Abstract
Introduction T cells engineered to express transgenic T cell receptors (TCRs) or chimeric antigen receptors (CARs) have emerged as powerful treatment options for some malignancies. However, while CAR T cells have induced impressive initial response rates in patients with B-precursor acute lymphoblastic leukemia (ALL) and lymphoma, they fail to mediate long-term relapse-free survival in 40-60% of patients and have not been successful in most solid tumors. For both TCR- and CAR-based approaches, T cell persistence and long-term functionality can be diminished by chronic antigen stimulation or tonic signaling. One approach to enhance the efficacy of cell therapies that has been explored by several groups is the overexpression of specific transcription factors (e.g. AP-1/ATF transcription factors such as c-JUN or BATF); however, more systematic gain-of-function screens in human CAR T cells have not yet been done.
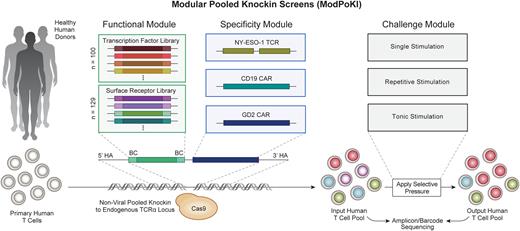
Methods and Results Here, we introduce CRISPR-based modular pooled knockin (ModPoKI) screens to evaluate hundreds to thousands of different constructs in human T cells and their potential to overcome TCR or CAR T cell dysfunction. We generated two barcoded ModPoKI libraries of 100 transcription factors (TFs) and 129 natural and synthetic surface receptors (SRs) to identify those that confer a fitness advantage to TCR or CAR T cells. The libraries were combined with either an NY-ESO-1-specific TCR, an anti-CD19 CAR or a high affinity anti-GD2 CAR that is known to induce tonic signaling-based dysfunction. The libraries and the TCR/CAR were engineered as polycistronic sequences that were non-virally integrated into the TRAC (T cell receptor alpha chain constant) locus of primary human T cells.
We performed >20 unique ModPoKI screens including bead-based single stimulation, repetitive stimulation with target cells and tonic stimulation (GD2 CAR model). While constructs containing known AP-1/ATF transcription factors BATF and BATF3 showed increased abundance across multiple screens, transcription factor AP4 (TFAP4) knockin (KI) constructs were more clearly enriched after repetitive stimulation and tonic signaling, suggesting potentially undescribed benefits in exhaustion-prone environments.
We next validated single knockins of TFAP4 or a control construct (truncated nerve growth factor receptor, tNGFR) in combination with the GD2 or the CD19 CAR. TFAP4 KI led to increased levels of cytokine release, proliferation and target cell killing compared to conventional CAR T cells. Moreover, TFAP4 KI mediated increased tumor control and survival in an in vivo model. Interestingly, we observed elevated levels of CD25 (IL2RA) expression on TFAP4 KI T cells and enrichment of genes related to IL2/STAT5 pathways which could hint at a potential benefit in situations of IL-2 competition/presence of regulatory T cells.
Lastly, we aimed to discover specific combinations of TFs that work in concert to enhance T cell fitness in the setting of tonic CAR signaling. We created a ~10,000-member library (102 x 102 - pairwise combinations of 100 transcription factors plus two controls) cloned into constructs with the GD2 CAR and performed a tonic signaling ModPoKI screen. Analysis of the constructs that increased the most in relative abundance highlighted that several of the top performing constructs included either TFAP4, BATF, BATF3 or a combination of TFAP4 and BATF(3), suggesting that TFAP4 and BATF(3) are key transcription factors that can coordinately drive increased T cell fitness upon repetitive stimulation. Validation analyses confirmed that the combinatorial KI of a BATF-TFAP4 construct confers highest cytotoxic capacity in vitro and in vivo compared to single knockin (BATF-RFP or RFP-TFAP4) or control knockin (RFP-tNGFR) GD2 CAR constructs.
Conclusions In conclusion, ModPoKI screens allow for customizable parallel evaluation and functional characterization of hundreds to thousands of different T cell constructs. Using clinically relevant screening modalities, we nominated candidate genes that can synthetically re-write the T cell response to repetitive antigen exposure or tonic signaling and thus have the potential to program more effective TCR or CAR T cell therapies for cancer.
Disclosures
Blaeschke:Arsenal Biosciences: Patents & Royalties; The Bristol Myers Squibb Foundation: Other: Research award; Gilead and Kite: Other: Research award. Mowery:Andreesen Horowitz: Other: Compensated Bio+Health Venture Fellow. Schmidt:Arsenal Biosciences: Consultancy. Goodman:NExTNet: Consultancy, Current equity holder in private company; Manifold Bio: Consultancy, Current equity holder in private company; Gordian Biotechnology: Consultancy, Current equity holder in private company; Retro Biosciences: Consultancy, Current equity holder in private company; Arsenal Biosciences: Current equity holder in private company. Eyquem:Takeda: Research Funding; Casdin Capital: Consultancy; Cytovia Therapeutics: Consultancy, Current equity holder in private company, Research Funding; Mnemo Therapeutics: Current equity holder in private company, Other: Compensated co-founder. Roth:Arsenal Biosciences: Membership on an entity's Board of Directors or advisory committees, Other: Compensated co-founder. Marson:Anthem: Research Funding; EPIQ: Other: Client; Arsenal Biosciences: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Other: Compensated co-founder; Spotlight Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Other: Compensated co-founder; Survey Genomics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Other: Co-founder; NewLimit: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; PACT Pharma: Current equity holder in private company, Other: Speaking and/or advising fees; 23andMe: Other: Speaking and/or advising fees; Juno Therapeutics: Other: Speaking and/or advising fees, Research Funding; Trizell: Other: Speaking and/or advising fees; Vertex: Other: Speaking and/or advising fees; Merck: Divested equity in a private or publicly-traded company in the past 24 months, Other: Speaking and/or advising fees; Amgen: Other: Speaking and/or advising fees; Genentech: Other: Speaking and/or advising fees; AlphaSights: Other: Speaking and/or advising fees; Rupert Case Management: Other: Speaking and/or advising fees; Bernstein: Other: Speaking and/or advising fees; ALDA: Other: Speaking and/or advising fees; Gilead: Research Funding; GlaxoSmithKline: Research Funding; Sanofi: Research Funding; Epinomics: Research Funding; Offline Ventures: Other: Investor, informal advisor.
Author notes
Asterisk with author names denotes non-ASH members.